Lactones in plants and supplements - types, properties and medical perspectives

Just as there are a multitude of substances circulating in our bodies that affect health, the same is true of plants. Many components of plants have a positive effect not only on themselves, but also on us when we consume them. We are well aware of such components as polyphenols, flavonoids, alkaloids, sterols. Lactones are less talked about, but this should not detract from their properties. Scientists have long been developing new drugs to help patients with many ailments thanks to the inspiration of lactones. Also, in some very popular supplements like Ginkgo biloba and Ashwagandha we find lactones, which are responsible for their health-promoting effects. In this article, we will discuss what types of lactones are found in nature, where they occur and what they are responsible for, as well as what we can expect from them in the future. Read to the end!
- What are lactones? Definition and properties
- Examples of natural lactones that are used in dietary supplements
- Division and classification of lactones
- Applications of lactones in industry
- Summary
What are lactones? Definition and properties
Lactones are biologically active compounds found in many plants, but also in fungi, bacteria, marine sponges and other organisms. Many of them are aromatic substances that give the plant its smell and sometimes its taste. However, they are not as small and volatile as the substances contained in essential oils. In them, only small amounts of lactones can be contained, as their mass is a tad too large to pass efficiently in the course of distillation. Instead, you can enjoy the aroma of lactones by choosing absolutes (such as jasmine) or pressed essential oils (such as bergamot) instead of distilled ones.
Chemically, they can be classified as intramolecular cyclic esters of hydroxy acids with different ring sizes[1]. Due to the stability of the ring structure, the most common are γ- and δ-lactones with five- and six-membered rings. The Greek letters are just used to denote the size of the lactone ring. So, for example, all γ-lactones have five-membered rings, and all ε-lactones have seven-membered rings.
Compounds with a lactone fragment can have completely different carbon skeletons and can be assigned to different classes of compounds, such as sesquiterpene lactones, coumarins or steroid skeleton lactones.
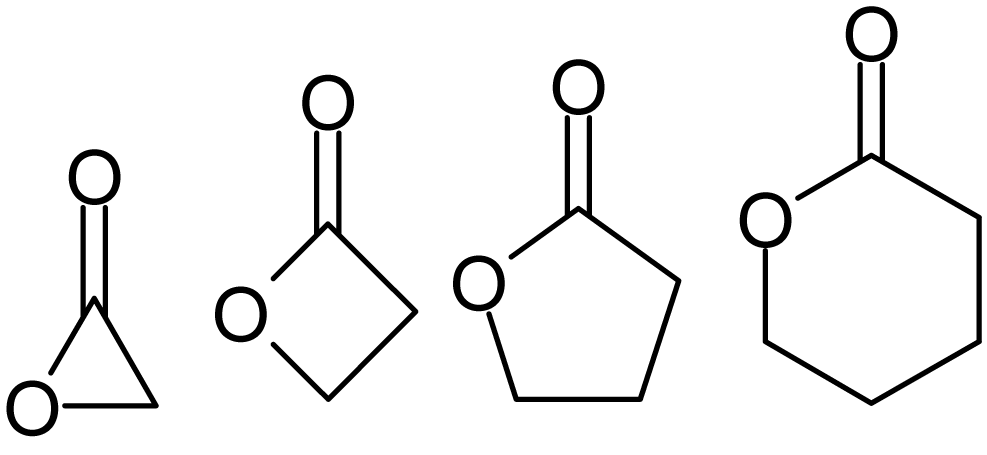
The chemical skeletons are successively α-, β-, γ-, δ-lactones.
The smallest compounds in this class are α-, β-, γ-, δ- and ω-lactones. They contain 3-, 4-, 5-, 6- and 7-membered rings, respectively. The high stability of lactone rings defines the widespread occurrence of γ- and δ- lactones in nature. It is estimated that more than 3,000 γ-lactones occur naturally. α-lactones, on the other hand, can only be obtained synthetically[2].
The diversity makes this an extremely interesting group that exhibits a number of important chemical and biological properties[1]. Among these activities, the most commonly mentioned are:
- cytotoxic,
- anti-inflammatory,
- antimalarial,
- antiviral,
- antimicrobial.
Lactones and their derivatives are seen as candidates for filling the gap in pharmacology created by the spread of pathogenic bacteria resistant to conventional antibiotics. At the same time, it is worth mentioning that lactones have long functioned in medicine. For example, the antibiotic erythromycin has a 14-membered lactone ring in addition to other functional groups.
Examples of natural lactones that are used in dietary supplements
Let's start with practical information, that is, which lactones can be taken in the form of dietary supplements to realistically affect your health. You will learn the classification and chemical details later in the article.
Terpene lactones
Ginkgo biloba extracts are one of the main sources of lactones in supplementation. Standard extracts are standardized to contain 6% terpene lactones. These lactones consist of bilobalide and ginkgolides, the main active substances in ginkgo, responsible for most of its health properties[3]. Ginkgolides are diterpene lactones, while bilobalide is a triterpene lactone (more specifically, a sesquiterpene trilactone). In the total pool of lactones, ginkgolides comprise 3.1 and bilobalide 2.9 percentage points.
Vitanolides
This is the main group of active substances in the famous adaptogenic plant Vitania sluggard, known as Ashwagandha. Its main domain is adaptogenic activity, i.e. increasing resistance to stressful conditions. However, the spectrum of health-promoting activities is much broader.
Vitanolides are a group of naturally occurring lactone triterpenoids[4]. The health-promoting properties of lactones from Ashwagandha are so valuable that numerous attempts are being made to synthesize them and create derivatives with even greater potential.
Artemisinin
Artemisinin is a sesquiterpene lactone isolated from mugwort(Artemisia annua). It is used in supplements to support the immune system and as an anti-inflammatory agent.
According to studies, artemisinin is effective against both drug-resistant and cerebral strains of Plasmodium falciparum that cause malaria[5].
Many more different sesquiterpene lactones are contained in the Asteraceae family of plants, especially in species of the genus Artemisia [6], not just artemisinin. The antimicrobial activity of sesquiterpenes may be related to changes in protein synthesis, altered cell permeability or interaction with cell wall phospholipids disrupting membrane integrity[1].
Ecdysteroids
Plants such as Ajuga turkestanica, Leuzea carthamoides and Spinacia oleracea (i.e., common spinach) contain ecdysteroids, which have a lactone structure. Examples include ajugalactone, or cartamosterone[7].
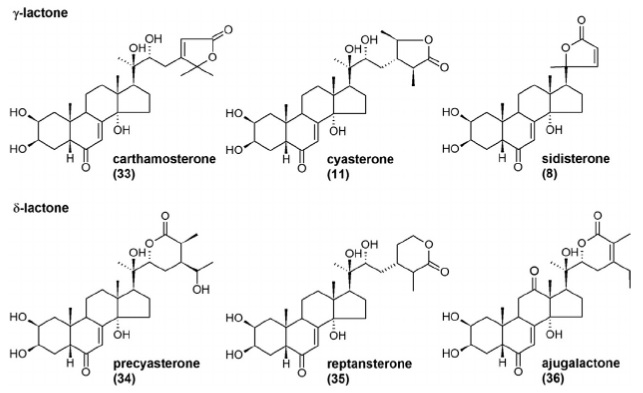
Graphic source - doi: 10.1016/B978-0-444-63473-3.00005-8.
Kavalactones
These are active substances contained in methistine pepper, or kava kava. In Poland, unfortunately, Kava kava is not approved for use in food products, but in the US, for example, kava kava extracts in capsules with standardized kavalactones are available for purchase[8]. There are many different substances among them, but 6 are listed as the main ones - their skeletons are shown in the graphic below.
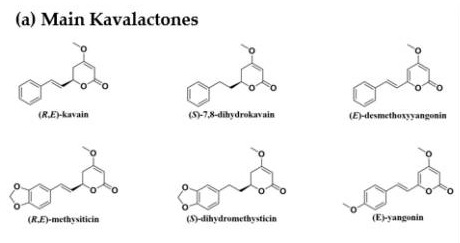
Graphic source - doi: 10.3390/nu12103044
Monacolin K
Chemically, monacolin K has a lactone structure, containing an ester ring. Its action is analogous to that of synthetic statins, and when converted to the active acid form, it becomes an inhibitor of the enzyme responsible for cholesterol synthesis in the body (it is an HMG-CoA reductase inhibitor)[9]. The EFSA panel concluded that monacolin K in lactone form is identical to lowastatin, the active ingredient in several medicinal products approved for the treatment of hypercholesterolemia in the EU. For this reason, regulations have limited the allowable daily intake of monacolin K from supplements to doses of less than 3 mg.
Division and classification of lactones
We will briefly review the most numerous and common groups of lactones.
[this section is a bit boring, but the brief included chemical information, and here there is a heavy saturation of characteristic keywords; I would do it in the form of a drop-down accordion, the way faq sections are done].
γ-lactones
These are 5-membered lactones. γ-lactones are present in the structure of several natural products, such as γ-saturated butyrolactone and α-β-unsaturated butenolides. Butenolides exhibit fascinating biological properties, for example[2]:
- 3-methyl-2H-furo[2,3-c]pyran-2-one exhibits strong herbicidal activity,
- flupiradifuron is an effective insecticide,
- rubrolidium M and basidalin show antitumor properties,
- 5-octylfuran-2(5H)-one shows antifouling activity.
Butenolides and butyrolactones are also known for their characteristic odor, making them highly used in the food and perfume industries.
δ-lactones
6-membered lactones. They have a wide representation in nature. Like γ-lactones, δ-lactones can be divided into saturated and α-β-unsaturated lactones, the latter being the most common. The δ-lactone fragment is common in several natural compounds with diverse biological activities[2], such as:
- HIV protease inhibition,
- induction of apoptosis (goniotalamin and rasfonin),
- Anti-leukemic activity (dictyopyrone C),
- anticancer (pyronetin),
- leishmanicidal,
- trypanicidal,
- antifungal (argentilactone),
- antimicrobial,
- anti-inflammatory.
Medium-sized lactones
These lactones contain rings containing 8 to 11 members. The most abundant in nature are 10-member decalactones. Among natural 8-member lactones, we distinguish octalactones A and B. They have potential cytotoxic activity against melanoma and colon tumor cells. Cephalosporolides and solandelactones are other examples of naturally occurring 8-membered lactones[2].
Oxylipins, halicholactone, neohalicholactone and topsentolides are some representatives of 9-member lactones. Seven topsentolides (A1, A2, B1, B2, B3, C1 and C2) showed moderate cytotoxic activity against human cancer cell lines. Antimycin dilactones are known for their antibiotic and antifungal properties and their ability to induce apoptosis of cancer cells. Decalactones, or nonanolides, are the best-studied natural medium-sized lactones and exhibit a variety of biological properties. Decaestrin is distinguished by its inhibitory effect on cholesterol biosynthesis.
Phthalides
Phthalides are bicyclic molecules formed by the combination of a γ-lactone and a benzene ring. They are important organic molecules produced by several genera of plants in the Apiaceae family. These compounds are also found in the Asteraceae, Acanthaceae, Amaranthaceae and Magnoliaceae.
This class of compounds has been studied since the 18th century, and there are numerous reports in the literature on its various ethnobotanical uses and biological activities. For example:
- (S)-3-n-butylphthalide is marketed as an anticonvulsant drug for the treatment of cerebral ischemia,
- isopestacin has antifungal and antioxidant properties,
- fuscinarin exerts anti-amnesic effects,
- (±)-concentricolide has antiviral activity.
Phthalides also exhibit antimicrobial, antiplatelet and analgesic properties.
Coumarins
These are plant and fungal secondary metabolites. These heterocyclic compounds contain a benzene ring linked to an α-pyrone. They attract attention for their antiviral, anticancer, Alzheimer's prevention, antidepressant, antibiotic (armillarin), anti-inflammatory (scopoletin) and anticoagulant (warfarin and dicumarol) effects.
Coumarins are widely used in the cosmetic, dye and food industries and as luminescent materials.
Furanocoumarins
Compounds containing a furan ring linked to the coumarin skeleton. They are produced by plants in response to stress and as protection against predators such as fungi, bacteria and insects. Furanocoumarins are used to treat skin diseases.
Spirolactones
These are naturally occurring compounds that comprise a large family of structurally diverse molecules with a wide range of biological activities:
- antimicrobial (spiroindicumide A),
- Antifungal (perrenniporide A),
- anti-inflammatory (abiespiroside A),
- antiparasitic (plumericin),
- antiviral (biyouyanagin B),
- cytotoxic (yaoshanenolide A).
In addition to naturally occurring compounds, spirolactones include synthetic steroids known as 17α-spirolactones. The best-known member of this class is spironolactone due to its antagonism to aldosterone, a hormone in the renin-angiotensin-aldosterone system associated with hypertension, cardiac hypertrophy and cardiovascular fibrosis.
Strigolactones
These are phytohormones isolated from plants in the genera Striga and Orobanche. Strigolactones stimulate seed germination of parasitic plants upon detection of a host, regulate plant growth, inhibit germination and mediate underground chemical communication between plants and neighboring organisms. The best-known natural examples have a tricyclic ABC core with γ-lactone as one of the rings and an exocyclic D ring of butenolide. This structure can be found in 5-deoxystrigol.
Strigolactones are used as soil additives to control weeds, thereby reducing agricultural losses associated with weed damage. These compounds induce suicidal germination. That is, signals emitted by the presence of isolated rhizosphere compounds or synthetic equivalents trigger the germination of parasitic seeds without the presence of a host, leading to the death of the weeds.
Macrolactones
Macrolactones or macrocyclic lactones contain rings with 12 or even more members. They can be classified as olides or diolides.
Kukuolids act as pheromones and are used by many animals for chemical communication. Kukuolid X has been identified as a pheromone that attracts the female beetle Oryzaephilus surinamensis, a pest of corn and rice.
Macrolactins are macrolides isolated from strains of Bacillus and Actinomadura bacteria. Currently, 19 macrolactins (A-S) have been described in the literature. Their biological activity ranges from antiviral to antifungal. For example, macrolactin A has strong activity against herpes simplex virus and can control HIV replication in humans. Macrolactins T and B, on the other hand, are capable of inhibiting the fungi Alternaria solani and Pyricularia oryzae and the bacterium Staphylococcus aureus. Then there are quinolidomycins A1, A2 and B1, of which quinolidomycins A1 and B1 inhibit the growth of various cancer cells, including drug-resistant ones.
Motivated by the numerous examples of naturally occurring lactones and their wide range of biological activities, chemists associated with organic chemistry have turned their attention to the design of lactone rings.
Applications of lactones in industry
Lactones are such interesting substances that it would be a disservice not to exploit their potential. For this reason, they are used in various industries.
Lactones are aromatic compounds, so the perfume industry is one of the strongest beneficiaries. The sensory properties of lactones make it possible to obtain unusual aromas, such as the scent of jasmine, vanilla or catnip. Various substances with lactone structures can be found on the labels of perfumes and perfumed cosmetics.
Wide applications have also been found in agriculture and horticulture. Some of the lactones have very efficient activity against insects, parasites, or weeds.
Summary
The family of lactones includes thousands of different substances. Although they share a common element in their structure (the lactone ring), they can differ drastically in terms of action. They do not receive as much attention in popular science texts as, for example, polyphenols, but there are still many lactones that have outstanding effects on human health. It is thanks to lactones that we owe the benefits of herbs such as Ashwagandha and Gingo biloba. The presence of lactones in nature and the conduct of more research on them opens the door for scientists to gain new tools to develop newer and better drugs that save people's lives. We are keeping our fingers crossed that we will be able to benefit from the power of lactones as much as possible!
Sources:
 ⮜ Previous article
⮜ Previous article
Spontaneous activity - why is it important?
 Next article ⮞
Next article ⮞


